Samarium(II) iodide
 | |
| Names | |
|---|---|
| IUPAC name samarium(II) iodide | |
| Other names samarium diiodide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | SmI2 |
| Molar mass | 404.16 g/mol |
| Appearance | green solid |
| Melting point | 520 °C (968 °F; 793 K) |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions | Samarium(II) chloride Samarium(II) bromide |
Other cations | Samarium(III) iodide Europium(II) iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |

Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and forms a dark blue solution in THF.[1] It is a strong one-electron reducing agent that is used in organic synthesis.
Structure
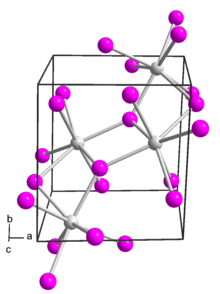
In solid samarium(II) iodide, the metal centers are seven-coordinate with a face-capped octahedral geometry.[2]

In its ether adducts, samarium remains heptacoordinate with five ether and two terminal iodide ligands.[3]
Preparation
Samarium iodide is easily prepared in nearly quantitative yields from samarium metal and either diiodomethane or 1,2-diiodoethane.[4] When prepared in this way, its solutions is most often used without purification of the inorganic reagent.
| () |
Solid, solvent-free SmI2 forms by high temperature decomposition of samarium(III) iodide (SmI3).[5][6][7]
Reactions
Samarium(II) iodide is a powerful reducing agent – for example it rapidly reduces water to hydrogen.[2] It is available commercially as a dark blue 0.1 M solution in THF. Although used typically in superstoichiometric amounts, catalytic applications have been described.[8]
Organic chemistry
Samarium(II) iodide is a reagent for carbon-carbon bond formation, for example in a Barbier reaction (similar to the Grignard reaction) between a ketone and an alkyl iodide to form a tertiary alcohol:[9]
- R1I + R2COR3 → R1R2C(OH)R3

Typical reaction conditions use SmI2 in THF in the presence of catalytic NiI2.
Esters react similarly (adding two R groups), but aldehydes give by-products. The reaction is convenient in that it is often very rapid (5 minutes or less in the cold). Although samarium(II) iodide is considered a powerful single-electron reducing agent, it does display remarkable chemoselectivity among functional groups. For example, sulfones and sulfoxides can be reduced to the corresponding sulfide in the presence of a variety of carbonyl-containing functionalities (such as esters, ketones, amides, aldehydes, etc.). This is presumably due to the considerably slower reaction with carbonyls as compared to sulfones and sulfoxides. Furthermore, hydrodehalogenation of halogenated hydrocarbons to the corresponding hydrocarbon compound can be achieved using samarium(II) iodide. Also, it can be monitored by the color change that occurs as the dark blue color of SmI2 in THF discharges to a light yellow once the reaction has occurred. The picture shows the dark colour disappearing immediately upon contact with the Barbier reaction mixture.
Work-up is with dilute hydrochloric acid, and the samarium is removed as aqueous Sm3+.
Carbonyl compounds can also be coupled with simple alkenes to form five, six or eight membered rings.[10]
Tosyl groups can be removed from N-tosylamides almost instantaneously, using SmI2 in conjunction with distilled water and an amine base. The reaction is even effective for deprotection of sensitive substrates such as aziridines:[11]

Removal of a tosyl group from an N-tosylamide using SmI2
In the Markó-Lam deoxygenation, an alcohol could be almost instantaneously deoxygenated by reducing their toluate ester in presence of SmI2.

Markó-Lam deoxygenation using SmI2
The applications of SmI2 have been reviewed.[12][13][14] The book Organic Synthesis Using Samarium Diiodide, published in 2009, gives a detailed overview of reactions mediated by SmI2.[15]
References
- ^ https://www.sigmaaldrich.com/GB/en/sds/aldrich/347116?userType=anonymous [bare URL]
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ William J. Evans; Tammy S. Gummersheimer & Joseph W. Ziller (1995). "Coordination Chemistry of Samarium Diiodide with Ethers Including the Crystal Structure of Tetrahydrofuran-Solvated Samarium Diiodide, SmI2(THF)5". J. Am. Chem. Soc. 117 (35): 8999–9002. doi:10.1021/ja00140a016.
- ^ P. Girard, J. L. Namy and H. B. Kagan (1980). "Divalent Lanthanide Derivatives in Organic Synthesis. 1. Mild Preparation of SmI2 and YbI2 and Their Use as Reducing or Coupling Agents". J. Am. Chem. Soc. 102 (8): 2693–2698. doi:10.1021/ja00528a029.
- ^ G. Jantsch, N. Skalla: "Zur Kenntnis der Halogenide der seltenen Erden. IV. – Über Samarium(II)jodid und den thermischen Abbau des Samarium(III)jodids", Zeitschrift für Allgemeine und Anorganische Chemie, 1930, 193, 391–405; doi:10.1002/zaac.19301930132.
- ^ G. Jantsch: "Thermischer Abbau von seltenen Erd(III)halogeniden", Die Naturwissenschaften, 1930, 18 (7), 155–155; doi:10.1007/BF01501667.
- ^ Gmelins Handbuch der anorganischen Chemie, System Nr. 39, Band C 6, p. 192–194.
- ^ Huang, Huan-Ming; McDouall, Joseph J. W.; Procter, David J. (2019). "SmI2-catalysed cyclization cascades by radical relay". Nature Catalysis. 2 (3): 211–218. doi:10.1038/s41929-018-0219-x. S2CID 104423773.
- ^ Machrouhi, Fouzia; Hamann, Béatrice; Namy, Jean-Louis; Kagan, Henri B. (1996). "Improved Reactivity of Diiodosamarium by Catalysis with Transition Metal Salts". Synlett. 1996 (7): 633–634. doi:10.1055/s-1996-5547. S2CID 196761752.
- ^ Molander, G. A.; McKiie, J. A. (1992). "Samarium(II) iodide-induced reductive cyclization of unactivated olefinic ketones. Sequential radical cyclization/intermolecular nucleophilic addition and substitution reactions". J. Org. Chem. 57 (11): 3132–3139. doi:10.1021/jo00037a033.
- ^ Ankner, Tobias; Göran Hilmersson (2009). "Instantaneous Deprotection of Tosylamides and Esters with SmI2/Amine/Water". Organic Letters. 11 (3). American Chemical Society: 503–506. doi:10.1021/ol802243d. PMID 19123840.
- ^ Patrick G. Steel (2001). "Recent developments in lanthanide mediated organic synthesis". J. Chem. Soc., Perkin Trans. 1 (21): 2727–2751. doi:10.1039/a908189e.
- ^ Molander, G. A.; Harris, C. R. (1996). "Sequencing Reactions with Samarium(II) Iodide". Chem. Rev. 96 (1): 307–338. doi:10.1021/cr950019y. PMID 11848755.
- ^ K. C. Nicolaou; Shelby P. Ellery; Jason S. Chen (2009). "Samarium Diiodide Mediated Reactions in Total Synthesis". Angew. Chem. Int. Ed. 48 (39): 7140–7165. doi:10.1002/anie.200902151. PMC 2771673. PMID 19714695.
- ^ Procter, David J.; Flowers,II, Robert A.; Skydstrup, Troels (2009). Organic Synthesis Using Samarium Diiodide. Royal Society of Chemistry. ISBN 978-1-84755-110-8.
- v
- t
- e
- Sm(CH3COO)3
- SmAs
- SmAsO4
- SmCl3
- SmBr3
- SmF3
- Sm(IO3)3
- SmI3
- Sm2O3
- SmOI
- Sm2S3
- Sm(OH)3
- SmN
- Sm(NO3)3
- SmPO4
- Sm2(MoO4)3
- SmSb
- Sm(ClO4)3
- Sm(C5H7O2)3
| Organosamarium(III) |
|
|---|
![{\displaystyle {\begin{array}{cl}{}\\{\ce {{Sm}+ ICH2I ->[{\ce {THF}}] SmI2}}+0.5{\ce {H2C=CH2}}\\{\ce {{Sm}+ I(CH2)2I ->[{\ce {THF}}] {SmI2}+ H2C=CH2}}\\{}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/97016669936673b8c455d5ddaaef4bb5c8ff5667)












