Surfactant protein D
| SFTPD | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SFTPD, COLEC7, PSP-D, SFTP4, SP-D, surfactant protein D | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 178635; MGI: 109515; HomoloGene: 2272; GeneCards: SFTPD; OMA:SFTPD - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Surfactant protein D, also known as SP-D, is a lung surfactant protein part of the collagenous family of lectins called collectin.[5] In humans, SP-D is encoded by the SFTPD gene[6][7] and is part of the innate immune system.[8][9] Each SP-D subunit is composed of an N-terminal domain, a collagenous region, a nucleating neck region, and a C-terminal lectin domain.[5][10] Three of these subunits assemble to form a homotrimer, which further assemble into a tetrameric complex.[5][10]
Interactions
Surfactant protein D has been shown to interact with DMBT1,[11][12] and hemagglutinin of influenza A virus.[13] Post-translational modification of SP-D i.e. S-nitrosylation switches its function.[14][15][16]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000133661 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000021795 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c Hoppe HJ, Barlow PN, Reid KB (May 1994). "A parallel three stranded alpha-helical bundle at the nucleation site of collagen triple-helix formation". FEBS Letters. 344 (2–3): 191–5. Bibcode:1994FEBSL.344..191H. doi:10.1016/0014-5793(94)00383-1. PMID 8187882. S2CID 2920539.
- ^ Rust K, Grosso L, Zhang V, Chang D, Persson A, Longmore W, et al. (October 1991). "Human surfactant protein D: SP-D contains a C-type lectin carbohydrate recognition domain". Archives of Biochemistry and Biophysics. 290 (1): 116–26. doi:10.1016/0003-9861(91)90597-C. PMID 1898081.
- ^ Lu J, Willis AC, Reid KB (June 1992). "Purification, characterization and cDNA cloning of human lung surfactant protein D". The Biochemical Journal. 284. 284 (3): 795–802. doi:10.1042/bj2840795. PMC 1132609. PMID 1339284.
- ^ "Entrez Gene: SFTPD surfactant, pulmonary-associated protein D".
- ^ Brandt EB, Mingler MK, Stevenson MD, Wang N, Khurana Hershey GK, Whitsett JA, Rothenberg ME (May 2008). "Surfactant protein D alters allergic lung responses in mice and human subjects". The Journal of Allergy and Clinical Immunology. 121 (5): 1140–1147.e2. doi:10.1016/j.jaci.2008.02.011. PMC 4145593. PMID 18355911.
- ^ a b Crouch E, Persson A, Chang D, Heuser J (June 1994). "Molecular structure of pulmonary surfactant protein D (SP-D)". The Journal of Biological Chemistry. 269 (25): 17311–9. doi:10.1016/S0021-9258(17)32556-5. PMID 8006040.
- ^ Tino MJ, Wright JR (April 1999). "Glycoprotein-340 binds surfactant protein-A (SP-A) and stimulates alveolar macrophage migration in an SP-A-independent manner". American Journal of Respiratory Cell and Molecular Biology. 20 (4): 759–68. doi:10.1165/ajrcmb.20.4.3439. PMID 10101009. S2CID 29437779.
- ^ Holmskov U, Lawson P, Teisner B, Tornoe I, Willis AC, Morgan C, et al. (May 1997). "Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule". The Journal of Biological Chemistry. 272 (21): 13743–9. doi:10.1074/jbc.272.21.13743. PMID 9153228.
- ^ Goh BC, Rynkiewicz MJ, Cafarella TR, White MR, Hartshorn KL, Allen K, et al. (November 2013). "Molecular mechanisms of inhibition of influenza by surfactant protein D revealed by large-scale molecular dynamics simulation". Biochemistry. 52 (47): 8527–38. doi:10.1021/bi4010683. PMC 3927399. PMID 24224757.
- ^ Guo CJ, Atochina-Vasserman EN, Abramova E, Foley JP, Zaman A, Crouch E, et al. (November 2008). "S-nitrosylation of surfactant protein-D controls inflammatory function". PLOS Biology. 6 (11): e266. doi:10.1371/journal.pbio.0060266. PMC 2581630. PMID 19007302.
- ^ Atochina-Vasserman EN, Guo CJ, Abramova E, Golden TN, Sims M, James ML, et al. (July 2015). "Surfactant dysfunction and lung inflammation in the female mouse model of lymphangioleiomyomatosis". American Journal of Respiratory Cell and Molecular Biology. 53 (1): 96–104. doi:10.1165/rcmb.2014-0224OC. PMC 4566108. PMID 25474372.
- ^ Atochina-Vasserman EN, Winkler C, Abramova H, Schaumann F, Krug N, Gow AJ, et al. (April 2011). "Segmental allergen challenge alters multimeric structure and function of surfactant protein D in humans". American Journal of Respiratory and Critical Care Medicine. 183 (7): 856–64. doi:10.1164/rccm.201004-0654OC. PMC 3086753. PMID 21131470.
Further reading
- Atochina-Vasserman EN, Beers MF, Kadire H, Tomer Y, Inch A, Scott P, et al. (December 2007). "Selective inhibition of inducible NO synthase activity in vivo reverses inflammatory abnormalities in surfactant protein D-deficient mice". Journal of Immunology. 179 (12): 8090–7. doi:10.4049/jimmunol.179.12.8090. PMC 4009628. PMID 18056350.
- Guo CJ, Atochina-Vasserman EN, Abramova E, Foley JP, Zaman A, Crouch E, et al. (November 2008). "S-nitrosylation of surfactant protein-D controls inflammatory function". PLOS Biology. 6 (11): e266. doi:10.1371/journal.pbio.0060266. PMC 2581630. PMID 19007302.
- Hansen S, Holmskov U (August 1998). "Structural aspects of collectins and receptors for collectins". Immunobiology. 199 (2): 165–89. doi:10.1016/s0171-2985(98)80025-9. PMID 9777404.
- Lu J, Willis AC, Reid KB (June 1992). "Purification, characterization and cDNA cloning of human lung surfactant protein D". The Biochemical Journal. 284. 284 ( Pt 3) (3): 795–802. doi:10.1042/bj2840795. PMC 1132609. PMID 1339284.
- Persson AV, Gibbons BJ, Shoemaker JD, Moxley MA, Longmore WJ (December 1992). "The major glycolipid recognized by SP-D in surfactant is phosphatidylinositol". Biochemistry. 31 (48): 12183–9. doi:10.1021/bi00163a030. PMID 1457414.
- Ogasawara Y, Kuroki Y, Akino T (October 1992). "Pulmonary surfactant protein D specifically binds to phosphatidylinositol". The Journal of Biological Chemistry. 267 (29): 21244–9. doi:10.1016/S0021-9258(19)36824-3. PMID 1400434.
- Rust K, Grosso L, Zhang V, Chang D, Persson A, Longmore W, et al. (October 1991). "Human surfactant protein D: SP-D contains a C-type lectin carbohydrate recognition domain". Archives of Biochemistry and Biophysics. 290 (1): 116–26. doi:10.1016/0003-9861(91)90597-C. PMID 1898081.
- Kuroki Y, Shiratori M, Ogasawara Y, Tsuzuki A, Akino T (November 1991). "Characterization of pulmonary surfactant protein D: its copurification with lipids". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1086 (2): 185–90. doi:10.1016/0005-2760(91)90006-4. PMID 1932100.
- Crouch E, Persson A, Chang D, Heuser J (June 1994). "Molecular structure of pulmonary surfactant protein D (SP-D)". The Journal of Biological Chemistry. 269 (25): 17311–9. doi:10.1016/S0021-9258(17)32556-5. PMID 8006040.
- Schaeffer E, Guillou F, Part D, Zakin MM (November 1993). "A different combination of transcription factors modulates the expression of the human transferrin promoter in liver and Sertoli cells". The Journal of Biological Chemistry. 268 (31): 23399–408. doi:10.1016/S0021-9258(19)49476-3. PMID 8226864.
- Kölble K, Lu J, Mole SE, Kaluz S, Reid KB (August 1993). "Assignment of the human pulmonary surfactant protein D gene (SFTP4) to 10q22-q23 close to the surfactant protein A gene cluster". Genomics. 17 (2): 294–8. doi:10.1006/geno.1993.1324. PMID 8406480.
- Crouch E, Persson A, Chang D (January 1993). "Accumulation of surfactant protein D in human pulmonary alveolar proteinosis". The American Journal of Pathology. 142 (1): 241–8. PMC 1886847. PMID 8424457.
- Crouch, E.; Rust, K.; Veile, R.; Donis-Keller, H.; Grosso, L. (February 1993). "Genomic organization of human surfactant protein D (SP-D). SP-D is encoded on chromosome 10q22.2-23.1". Journal of Biological Chemistry. 268 (4): 2976–2983. doi:10.1016/S0021-9258(18)53869-2. PMID 8428971.
- Rust K, Bingle L, Mariencheck W, Persson A, Crouch EC (February 1996). "Characterization of the human surfactant protein D promoter: transcriptional regulation of SP-D gene expression by glucocorticoids". American Journal of Respiratory Cell and Molecular Biology. 14 (2): 121–30. doi:10.1165/ajrcmb.14.2.8630261. PMID 8630261.
- Bonaldo MF, Lennon G, Soares MB (September 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Holmskov U, Lawson P, Teisner B, Tornoe I, Willis AC, Morgan C, et al. (May 1997). "Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule". The Journal of Biological Chemistry. 272 (21): 13743–9. doi:10.1074/jbc.272.21.13743. PMID 9153228.
- Botas C, Poulain F, Akiyama J, Brown C, Allen L, Goerke J, et al. (September 1998). "Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D". Proceedings of the National Academy of Sciences of the United States of America. 95 (20): 11869–74. Bibcode:1998PNAS...9511869B. doi:10.1073/pnas.95.20.11869. PMC 21732. PMID 9751757.
- Håkansson K, Lim NK, Hoppe HJ, Reid KB (March 1999). "Crystal structure of the trimeric alpha-helical coiled-coil and the three lectin domains of human lung surfactant protein D". Structure. 7 (3): 255–64. doi:10.1016/S0969-2126(99)80036-7. PMID 10368295.
- Holmskov U, Mollenhauer J, Madsen J, Vitved L, Gronlund J, Tornoe I, et al. (September 1999). "Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D". Proceedings of the National Academy of Sciences of the United States of America. 96 (19): 10794–9. Bibcode:1999PNAS...9610794H. doi:10.1073/pnas.96.19.10794. PMC 17962. PMID 10485905.
- Lausen M, Lynch N, Schlosser A, Tornoe I, Saekmose SG, Teisner B, et al. (November 1999). "Microfibril-associated protein 4 is present in lung washings and binds to the collagen region of lung surfactant protein D". The Journal of Biological Chemistry. 274 (45): 32234–40. doi:10.1074/jbc.274.45.32234. PMID 10542261.
- Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U (June 2000). "Localization of lung surfactant protein D on mucosal surfaces in human tissues". Journal of Immunology. 164 (11): 5866–70. doi:10.4049/jimmunol.164.11.5866. PMID 10820266.
- Zhang L, Ikegami M, Crouch EC, Korfhagen TR, Whitsett JA (June 2001). "Activity of pulmonary surfactant protein-D (SP-D) in vivo is dependent on oligomeric structure". The Journal of Biological Chemistry. 276 (22): 19214–9. doi:10.1074/jbc.M010191200. PMID 11278637.
External links
- Surfactant+Protein+D at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- v
- t
- e
-
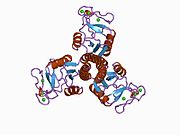 1b08: LUNG SURFACTANT PROTEIN D (SP-D) (FRAGMENT)
1b08: LUNG SURFACTANT PROTEIN D (SP-D) (FRAGMENT) -
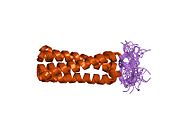 1m7l: Solution Structure of the Coiled-Coil Trimerization Domain from Lung Surfactant Protein D
1m7l: Solution Structure of the Coiled-Coil Trimerization Domain from Lung Surfactant Protein D -
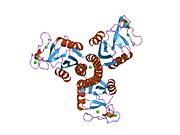 1pw9: High resolution crystal structure of an active recombinant fragment of human lung surfactant protein D
1pw9: High resolution crystal structure of an active recombinant fragment of human lung surfactant protein D -
 1pwb: High resolution crystal structure of an active recombinant fragment of human lung surfactant protein D with maltose
1pwb: High resolution crystal structure of an active recombinant fragment of human lung surfactant protein D with maltose -
 2ggu: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with maltotriose
2ggu: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with maltotriose -
 2ggx: Crystal structure of the trimer neck and carbohydrate recognition domain of human surfactant protein D in complex with p-nitrophenyl maltoside
2ggx: Crystal structure of the trimer neck and carbohydrate recognition domain of human surfactant protein D in complex with p-nitrophenyl maltoside -
 2orj: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with N-acetyl mannosamine
2orj: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with N-acetyl mannosamine -
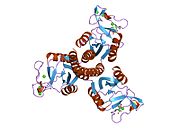 2ork: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with inositol-1-phosphate
2ork: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with inositol-1-phosphate -
 2os9: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with myoinositol
2os9: crystal structure of the trimeric neck and carbohydrate recognition domain of human surfactant protein D in complex with myoinositol
 | This protein-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e


























