Protein-coding gene in the species Homo sapiens
| PDLIM5 |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
2UZC, 2DAR |
|
|
| Identifiers |
|---|
| Aliases | PDLIM5, ENH, ENH1, LIM, L9, PDZ and LIM domain 5 |
|---|
| External IDs | OMIM: 605904; MGI: 1927489; HomoloGene: 21289; GeneCards: PDLIM5; OMA:PDLIM5 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 4 (human)[1] |
|---|
| | Band | 4q22.3 | Start | 94,451,857 bp[1] |
|---|
| End | 94,668,227 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 3 (mouse)[2] |
|---|
| | Band | 3|3 H1 | Start | 141,945,351 bp[2] |
|---|
| End | 142,101,457 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - Skeletal muscle tissue of biceps brachii
- Skeletal muscle tissue of rectus abdominis
- right ventricle
- triceps brachii muscle
- glutes
- thoracic diaphragm
- Epithelium of choroid plexus
- muscle of thigh
- gastrocnemius muscle
- body of tongue
|
| | Top expressed in | - soleus muscle
- myocardium of ventricle
- atrioventricular valve
- muscle of thigh
- quadriceps femoris muscle
- vastus lateralis muscle
- interventricular septum
- sternocleidomastoid muscle
- tibialis anterior muscle
- medial head of gastrocnemius muscle
|
| | More reference expression data |
|
|---|
| BioGPS | 

 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - protein binding
- metal ion binding
- protein N-terminus binding
- actin binding
- actinin binding
- protein kinase C binding
- cadherin binding involved in cell-cell adhesion
- muscle alpha-actinin binding
| | Cellular component | - cytoplasm
- cell junction
- neuron projection
- postsynaptic membrane
- plasma membrane
- synapse
- actin cytoskeleton
- membrane
- postsynaptic density
- cytosol
- stress fiber
- Z discdkac
- filamentous actin
| | Biological process | - regulation of dendritic spine morphogenesis
- regulation of synapse assembly
- cell-cell adhesion
- cell growth involved in cardiac muscle cell development
- heart development
- actin cytoskeleton organization
- muscle structure development
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | NM_001011513
NM_001011515
NM_001011516
NM_001256425
NM_001256426
|
|---|
NM_001256427
NM_001256428
NM_001256429
NM_006457 |
| NM_001190852
NM_001190853
NM_001190854
NM_001190855
NM_001190856
|
|---|
NM_001190857
NM_019808
NM_019809
NM_022554 |
|
|---|
| RefSeq (protein) | NP_001011513
NP_001011515
NP_001011516
NP_001243354
NP_001243355
|
|---|
NP_001243356
NP_001243357
NP_001243358
NP_006448 |
| NP_001177781
NP_001177782
NP_001177783
NP_001177784
NP_001177785
|
|---|
NP_001177786
NP_062782
NP_062783
NP_072048 |
|
|---|
| Location (UCSC) | Chr 4: 94.45 – 94.67 Mb | Chr 3: 141.95 – 142.1 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
PDZ and LIM domain protein 5 is a protein that in humans is encoded by the PDLIM5 gene.[5][6]
The protein encoded by this gene is a LIM domain protein. LIM domains are cysteine-rich double zinc fingers composed of 50 to 60 amino acids that are involved in protein-protein interactions. LIM domain-containing proteins are scaffolds for the formation of multiprotein complexes. The proteins are involved in cytoskeleton organization, cell lineage specification, organ development, and oncogenesis. The encoded protein is also a member of the Enigma class of proteins, a family of proteins that possess a 100-amino acid PDZ domain in the N-terminus and 1 to 3 LIM domains in the C terminus. Multiple transcript variants encoding different isoforms have been found for this gene, although not all of them have been fully characterized.[6]
Interactions
PDLIM5 has been shown to interact with PRKCB1.[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000163110 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000028273 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Wu M, Li Y, Ji C, Xu J, Zheng H, Zou X, Gu S, Lou Y, Xie Y, Mao Y (September 2004). "Cloning and identification of a novel human gene PDLIM5, a homolog of AD-associated neuronal thread protein (AD7c-NTP)". DNA Seq. 15 (2): 144–7. doi:10.1080/10425170310001656756. PMID 15346770. S2CID 36916587.
- ^ a b "Entrez Gene: PDLIM5 PDZ and LIM domain 5".
- ^ Kuroda, S; Tokunaga C; Kiyohara Y; Higuchi O; Konishi H; Mizuno K; Gill G N; Kikkawa U (December 1996). "Protein-protein interaction of zinc finger LIM domains with protein kinase C". J. Biol. Chem. 271 (49): 31029–32. doi:10.1074/jbc.271.49.31029. ISSN 0021-9258. PMID 8940095.
Further reading
- Sudo K, Chinen K, Nakamura Y (1995). "2058 expressed sequence tags (ESTs) from a human fetal lung cDNA library". Genomics. 24 (2): 276–9. doi:10.1006/geno.1994.1616. PMID 7698749.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Kuroda S, Tokunaga C, Kiyohara Y, et al. (1997). "Protein-protein interaction of zinc finger LIM domains with protein kinase C". J. Biol. Chem. 271 (49): 31029–32. doi:10.1074/jbc.271.49.31029. PMID 8940095.
- Ueki N, Seki N, Yano K, et al. (1999). "Isolation, tissue expression, and chromosomal assignment of a human LIM protein gene, showing homology to rat enigma homologue (ENH)". J. Hum. Genet. 44 (4): 256–60. doi:10.1007/s100380050155. PMID 10429367.
- Nakagawa N, Hoshijima M, Oyasu M, et al. (2000). "ENH, containing PDZ and LIM domains, heart/skeletal muscle-specific protein, associates with cytoskeletal proteins through the PDZ domain". Biochem. Biophys. Res. Commun. 272 (2): 505–12. doi:10.1006/bbrc.2000.2787. PMID 10833443.
- Hartley JL, Temple GF, Brasch MA (2001). "DNA Cloning Using In Vitro Site-Specific Recombination". Genome Res. 10 (11): 1788–95. doi:10.1101/gr.143000. PMC 310948. PMID 11076863.
- Wiemann S, Weil B, Wellenreuther R, et al. (2001). "Toward a Catalog of Human Genes and Proteins: Sequencing and Analysis of 500 Novel Complete Protein Coding Human cDNAs". Genome Res. 11 (3): 422–35. doi:10.1101/gr.GR1547R. PMC 311072. PMID 11230166.
- Simpson JC, Wellenreuther R, Poustka A, et al. (2001). "Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing". EMBO Rep. 1 (3): 287–92. doi:10.1093/embo-reports/kvd058. PMC 1083732. PMID 11256614.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Maeno-Hikichi Y, Chang S, Matsumura K, et al. (2003). "A PKC epsilon-ENH-channel complex specifically modulates N-type Ca2+ channels". Nat. Neurosci. 6 (5): 468–75. doi:10.1038/nn1041. PMID 12665800. S2CID 19964052.
- Iwamoto K, Kakiuchi C, Bundo M, et al. (2004). "Molecular characterization of bipolar disorder by comparing gene expression profiles of postmortem brains of major mental disorders". Mol. Psychiatry. 9 (4): 406–16. doi:10.1038/sj.mp.4001437. PMID 14743183.
- Petroziello J, Yamane A, Westendorf L, et al. (2004). "Suppression subtractive hybridization and expression profiling identifies a unique set of genes overexpressed in non-small-cell lung cancer". Oncogene. 23 (46): 7734–45. doi:10.1038/sj.onc.1207921. PMID 15334068.
- Iwamoto K, Bundo M, Washizuka S, et al. (2004). "Expression of HSPF1 and LIM in the lymphoblastoid cells derived from patients with bipolar disorder and schizophrenia". J. Hum. Genet. 49 (5): 227–31. doi:10.1007/s10038-004-0136-5. PMID 15362566.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Wiemann S, Arlt D, Huber W, et al. (2004). "From ORFeome to Biology: A Functional Genomics Pipeline". Genome Res. 14 (10B): 2136–44. doi:10.1101/gr.2576704. PMC 528930. PMID 15489336.
- Niederländer N, Fayein NA, Auffray C, Pomiès P (2005). "Characterization of a new human isoform of the enigma homolog family specifically expressed in skeletal muscle". Biochem. Biophys. Res. Commun. 325 (4): 1304–11. doi:10.1016/j.bbrc.2004.10.178. PMID 15555569.
- Kato T, Iwayama Y, Kakiuchi C, et al. (2005). "Gene expression and association analyses of LIM (PDLIM5) in bipolar disorder and schizophrenia". Mol. Psychiatry. 10 (11): 1045–55. doi:10.1038/sj.mp.4001719. PMID 16044170.
- Rual JF, Venkatesan K, Hao T, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Horiuchi Y, Arai M, Niizato K, et al. (2006). "A polymorphism in the PDLIM5 gene associated with gene expression and schizophrenia". Biol. Psychiatry. 59 (5): 434–9. doi:10.1016/j.biopsych.2005.07.041. PMID 16213469. S2CID 22930796.
PDB gallery
-
1wf7: Solution structure of the PDZ domain of Enigma homologue protein -
2dar: Solution structure of first LIM domain of Enigma-like PDZ and LIM domains protein -
2uzc: STRUCTURE OF HUMAN PDLIM5 IN COMPLEX WITH THE C-TERMINAL PEPTIDE OF HUMAN ALPHA-ACTININ-1 |

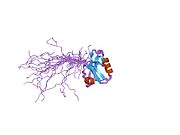 1wf7: Solution structure of the PDZ domain of Enigma homologue protein
1wf7: Solution structure of the PDZ domain of Enigma homologue protein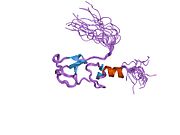 2dar: Solution structure of first LIM domain of Enigma-like PDZ and LIM domains protein
2dar: Solution structure of first LIM domain of Enigma-like PDZ and LIM domains protein 2uzc: STRUCTURE OF HUMAN PDLIM5 IN COMPLEX WITH THE C-TERMINAL PEPTIDE OF HUMAN ALPHA-ACTININ-1
2uzc: STRUCTURE OF HUMAN PDLIM5 IN COMPLEX WITH THE C-TERMINAL PEPTIDE OF HUMAN ALPHA-ACTININ-1






















